Consider a building under construction. You can imagine that the building consists of walls, and that the walls are made of bricks. Going into a little more detail, we see that the bricks are made of clay, and that the clay consists of small particles. So, what are clay particles made of? The simplest answer to this question is atoms. What is the structure of atoms? Can this structure be represented by a model? Humanity’s research into atomic models has been ongoing since ancient times. Moreover, this is one of the most beautiful adventures in the history of science and a wonderful example of how scientific developments occur. Let’s learn together about the steps of this ongoing adventure up to the present day.
Can Atoms Be Split?
Democritus Ancient people, just like people today, wondered what the objects they saw around them were made of. However, they did not have today’s technology. When they took a piece of wood and broke it, they ended up with smaller pieces of wood. They couldn’t go beyond that. Therefore, one of the most valid opinions was that no matter how much they broke the wood, they would still end up with wood. In 450 BC, Democritus said that matter could not be divided indefinitely, that there had to be an end to it. According to this idea, an object such as wood had to have an indivisible building block. This view was accepted, and the smallest building block of matter was called an “atom.” The word “atom” originated from the Ancient Greek word “atomos,” meaning “indivisible.” However, the only thing that has survived from Democritus to the present day is the word “atom.” Who Created the First Atomic Model? Scientific work on the atomic model began in the 19th century. The first person to scientifically examine the atom and its structure was the English chemist John Dalton.
Dalton proposed that all matter He proposed that atoms are composed of tiny particles called atoms. He discovered that all atoms of the same element are identical, while atoms of different elements are entirely different. He also stated that atoms cannot be broken down or recreated. According to him, atoms were solid spheres. In chemical reactions, the structure of atoms did not change; they could return to their original state. This model was not entirely accurate; because Dalton, like Democritus, said that the atom was indivisible and could not be broken down into smaller parts. Nevertheless, Dalton’s model was important because it was the first scientific model on the atom. With the technological capabilities of the time, this was all that could be said.
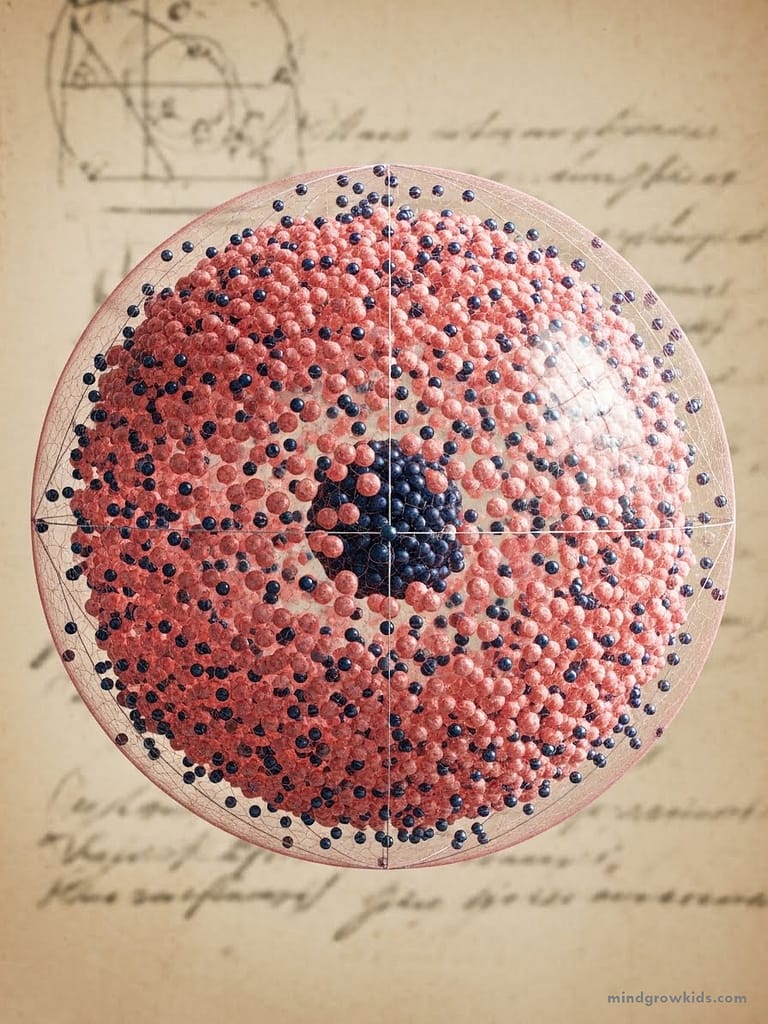
Where are the electrons in the raisin cake?
Like the Solar System…
New Zealand physicist Ernest Rutherford apparently didn’t like Thompson’s raisin cake, so he embarked on a new quest. Because it was so small, it was impossible to see the atom. Let’s imagine we are standing at the entrance of a dark cave. We don’t have a flashlight. To understand what’s inside the cave, let’s throw a stone in. We can get an idea about the cave from the sound it makes. For example, if there is a bear sleeping in the middle of the cave, it will wake up and let us know it’s there! At that time, the smallest known matter was alpha particles (these particles are the nucleus of a helium atom). It was known that alpha particles were positively charged. Certain natural minerals spontaneously emitted alpha particles, and alpha particles were used in taking X-ray films. Thompson took a very thin layer of gold and He sent alpha particles onto the plate. Around the plate was a screen that lit up when an alpha particle struck it. If the plum pudding model were correct, the alpha particles would have struck the atom and been reflected back. However, this did not happen.
Most of the particles passed through the plate. Rutherford hypothesized that since most alpha particles could pass through the plate, there must be large empty spaces in the atom’s structure. He also observed that some alpha particles were deflected, while others were reflected back. By studying these reflections and deflections, Rutherford proposed that at the center of the atom there was a nucleus composed of positive charges, surrounded by electrons. Electrons orbited the nucleus because of the attractive force between them. The only way to prevent the electrons from being drawn to the nucleus was for them to orbit the nucleus, just as the Earth orbits the Sun. This was a model similar to the Solar System. Although Rutherford’s model was quite successful, like all previous models, it also brought with it a number of problems. Nevertheless, Rutherford’s work earned him the Nobel Prize in Chemistry.
The Bohr Atomic Model:
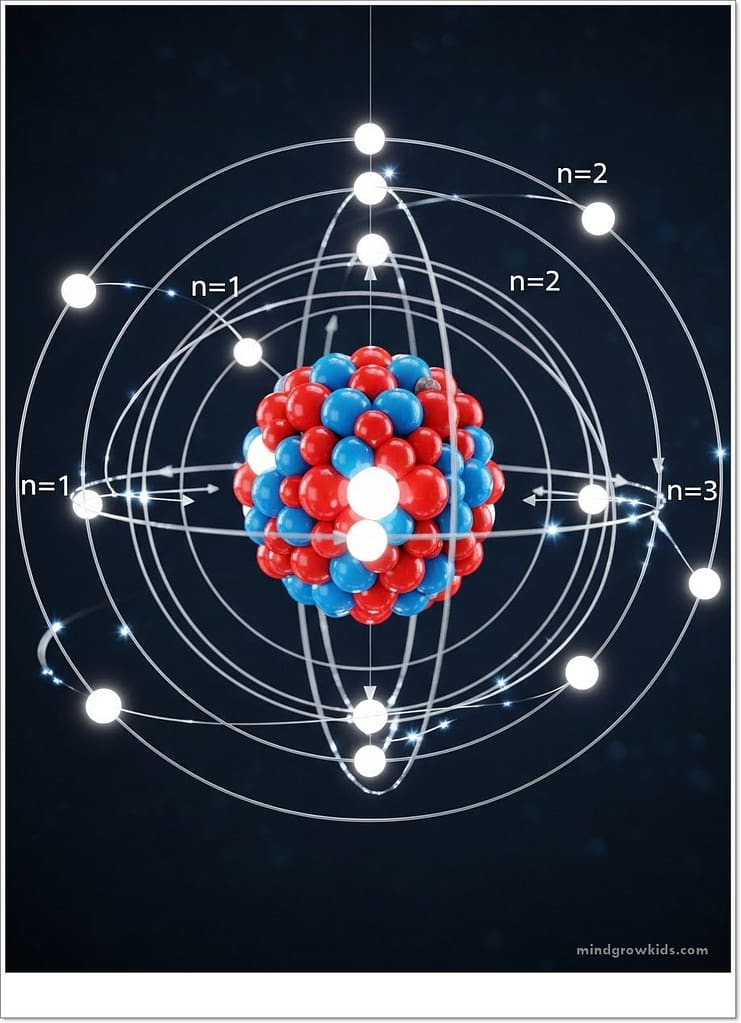
Until then, the laws of physics indicated that the rotation of a charged particle would cause it to lose energy. Our electron, however, rotates around the nucleus, yet its energy does not decrease. Niels Bohr, realizing this, came up with a completely new idea. The known laws of physics were insufficient to explain the atom! At this point, Bohr proposed the “Bohr Atomic Model,” which brought a new understanding to physics. This model stated that the known laws of physics were not valid for very small particles. This was a very important finding. This new approach, which matured with the work of other scientists on small particles, opened a new era in physics and became known as “quantum physics.” Quantum physics is still successfully used today for small particles. For example, the “nuclear magnetic resonance imaging” method used in imaging the body is an application of quantum physics. According to Bohr, electrons do not orbit the nucleus as they wish, but only in orbits at specific distances from the nucleus. Electrons at different distances have different energies. Therefore, these orbits are called “energy levels”. Electrons can only transition between these levels by changing their energy to the energy of that level. To give energy to an electron, light can be sent to the substance containing the electron, electricity can be applied, or the substance can be heated. The electron, however, can only give energy through radiation. Consider fluorescent lamps. They contain mercury vapor. When we apply electricity to the bulb, we transfer energy to the electrons of the mercury atoms, raising them to higher energy levels. They then release this energy through radiation to return to their original state. Because objects in nature don’t like high energy levels! Bohr applied these ideas to the simplest atom, the hydrogen atom. A hydrogen atom has only one proton and one electron. The electron orbits the proton. Bohr applied electricity to hydrogen gas, studied the emitted light, and measured the distances at which the electron could be found from the nucleus. This experiment showed that Bohr’s calculations and the model he created were correct. As a result, this work earned Bohr the Nobel Prize in Physics.
What Happened Next?
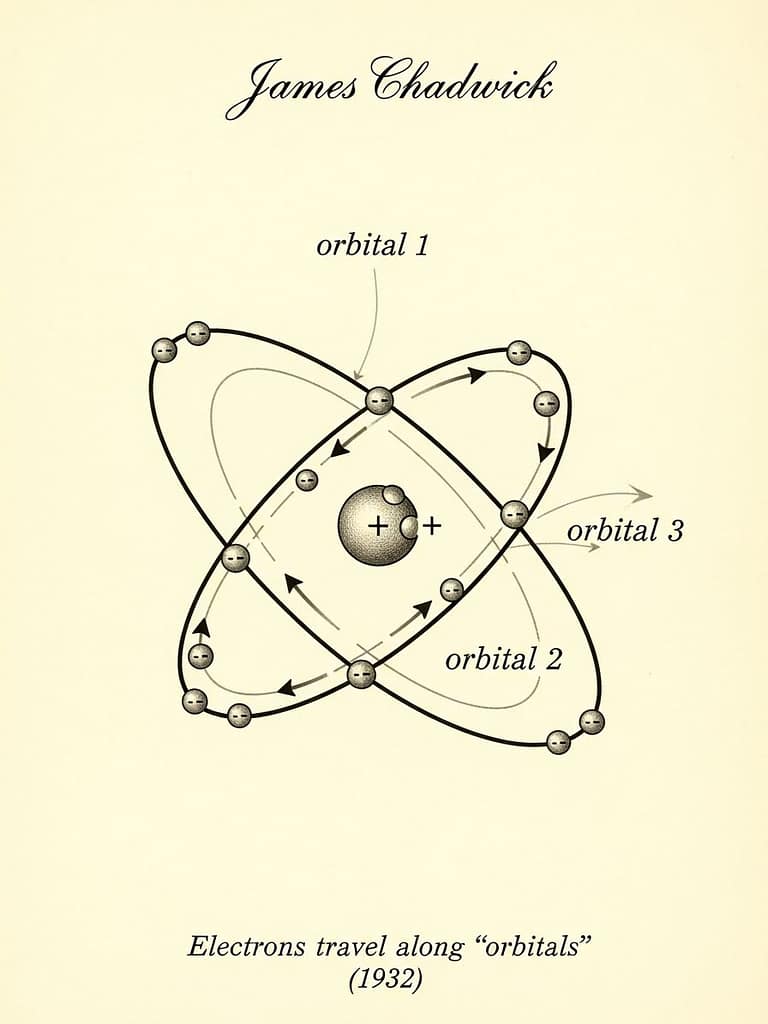
Since Bohr’s work, there have been several innovations in the atomic model. One of the most important of these was the discovery that the proton is not alone in the nucleus and has a companion called a “neutron.” The British physicist James Chadwick, who discovered this, received the Nobel Prize in Physics. While Bohr’s findings were correct, quantum physics, which deals with very small particles, revealed that we cannot know the exact positions of electrons around the nucleus. We could only know where they might be moving. These regions became known as “orbitals.” Imagine you have a cat at home; if you’re not home, you can’t know where the cat is, but you can guess where it might be. We can’t enter the atom ourselves, but we can predict where the electron might be located.
The Current Atomic Model
Today, we know that atoms are composed of electrons, protons, and neutrons. Protons and neutrons are located at the center, while electrons are found around the nucleus. Although we cannot know the exact positions of electrons, we can know where they might be. Furthermore, electron orbits are not circular. Electrons are much smaller than protons and neutrons. If we compare the size of an electron to a soccer ball, we can say that protons and neutrons are about the size of a soccer field! The masses of protons and neutrons are also much, much larger than those of electrons. Is that all? Of course not! So what makes up electrons, protons, and neutrons?!!
We now know that protons and neutrons are also made up of other particles called “quarks.” Based on current knowledge, electrons are considered one of the fundamental particles because they are indivisible. Scientists never stop and work to learn more. All these topics fall under the branch of physics called “particle physics.” Huge laboratories are being built to break down the atom and learn what’s inside. In these laboratories, particles are accelerated to great speeds and collided with each other. Just like colliding two boxes to break them open and look inside! All this work is to find the fundamental particles that make up the atom…